Early-life nutrition can profoundly affect development through the regulation of gene expression even when the DNA is not altered. In this blog, a research team out of University of Alabama at Birmingham summarize the evidence that an “epigenetics diet” can help protect against adverse effects of environmental pollution exposure whilst in utero and after birth.
Early-life dietary nutrition can profoundly affect individual developmental fate and disease prevention. For example, the presence of absence of royal jelly determines whether the larvae of female honey bees develop into a queen (presence of jelly) or a sterile worker (absence of jelly). In a recent review article published in Clinical Epigenetics, we have reported important roles of specific diets in protection of environmental pollution-induced genetic/epigenetic disorders through the regulation of gene expression even when the DNA is not altered.
Epigenetics may be one of the most important molecular mechanisms linking environmental stimulation, fetal programming, and adulthood phenotype.
Epigenetics and pollution
Epigenetics is an important additional aspect of the central dogma of molecular biology and reflects the interactions of the genome with its environment. Epigenetic changes affect gene expression without any changes to the underlying DNA sequence. The epigenome refers to the complete description of all epigenetic modifications across the genome, among which DNA methylation and histone modifications are the most important.
The mammalian epigenome experiences two major cycles of elimination and reconstruction including the periods of gametogenesis and early embryogenesis, during which the epigenome is vulnerable to environmental factors (see figure below).
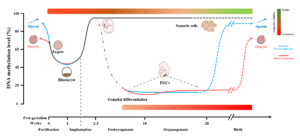
Environmental pollutions, including ambient air pollution (e.g. particulate matter, smoking, polycyclic aromatic hydrocarbons), hormone-disrupting chemicals (e.g. bisphenol A, vinclozolin, persistent organic pollutants) and heavy metals (e.g. arsenic, cadmium, lead) can seriously affect human health, especially during prenatal and early postnatal life.
The epigenetics diet
Tollefsbol’s lab coined the term “epigenetics diet” in 2011. It refers to a class of bioactive dietary compounds such as isothiocyanates in broccoli, genistein in soybean, resveratrol in red grapes and other commonly consumed foods, which have been shown to modify the epigenome leading to beneficial health outcomes. The epigenetics diet can inhibit tumor progression through modulation of epigenetic-modifying enzymes such as DNA methyltransferases and histone deacetylases as well as certain noncoding RNAs.

For our recent article, we reviewed recent advanced studies that some bioactive compounds may also counteract or attenuate the damage to the epigenome caused by environmental pollutions. For example, dietary supplementation with methyl donors (such as vitamin B12, folate, choline, and others) and the isoflavone genistein can reverse epigenome dysregulation induced by bisphenol A, a hormone-disrupting chemical of public health concern. B vitamins might avert the loss of DNA methylation induced by air pollution. Dietary folic acid supplementation has been shown to prevent the adverse effects caused by heavy metals.
We believe that epigenetic agents could be potentially used to counteract environmental pollution-induced epigenomic defects. In our article, we provide cautions about potential environmental toxins and chemicals in certain foods such as pesticides in fruits (e.g. strawberry) and leafy greens (e.g. spinach), bisphenol A in plastic containers of foods and beverages, dioxins in fatty foods, polycyclic aromatic hydrocarbons when meat is grilled or smoked at high temperatures and mercury in certain seafoods (e.g. king mackerel and swordfish). Those exposures, especially during early development, may lead to profound impacts on later-life health/disease consequences for the child via epigenetic mechanisms.
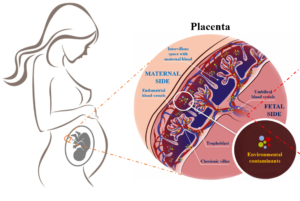
Epigenetics may be one of the most important molecular mechanisms linking environmental stimulation, fetal programming, and adulthood phenotype. Due to their reversible nature, epigenetic modifications are becoming an attractive therapeutic target.